{On the bottom of the page I list the burning flame colors when different elements are burning}.
So you have heard me speak of the fact that Retardants do nothing to a fire. Many like to regurgitate the same things they’ve been told over the past decades about retardants. “its not to stop the fire only to slow it down so we can get crews on it”, right!
Now, with the video above (Taken on the Jakes/Midas Fires 2025), pay particular attention to the fire behavor, around the 25 second mark into the video you can see the activity become less intense. At around the 43 second mark you can clearly see the entire line length become increasingly exothermic all at the same time. This is what I would expect one would observe when the temperatures have risen above the 500 deg C mark and the nitrogen is separating from the hydrogen in an oxygen atmosphere, that is in a NON Inert atmosphere.
So lets think about this for a moment shall we.
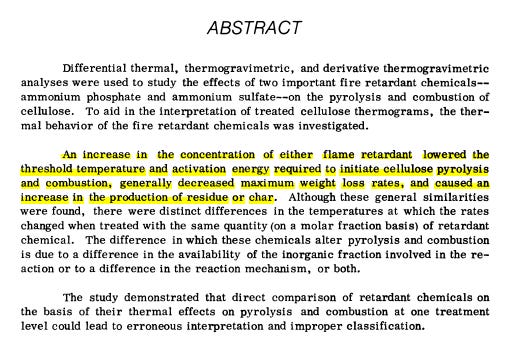
In 1972 Charles George, a research scientist for the USFS, produced a report titled. Effects of Ammonium Sulphate and Ammonium Phosphate on Flammability. He states in his introduction that the treating of fuels with retardants actually lower the required ignition temperatures. This is found for yourselves in the accompanying document.
The following Is stated in the abstract. (3rd line last paragraph).
This is further supported by yet another study done a year earlier in report INT-90 titled “The Effects of Ammonium Sulphate and Ammonium Phosphate on the pyrolisys and combustion of cellulose, 1971. the following screen shots are taken and reproduced here:
Now sofar this sounds great in theory, the problem is that 1. They are using fuel sample sizes that are literally .021” in diameter by 3” in length. So in my own opinion, of course the rates of spread would be greatly diminished, there’s nothing left fuel wise with that small of a sample size. 2. The Testing for the poyrolysis is done in an Inert Atmosphere. That is one that is devoid of “reactive gases” like oxygen. They do this to prevent unwanted reactions. Think about that!
* .021” is literally smaller than 1/16th of an inch (.0625), this equates to a fuel sample size that is actually 2.97 times SMALLER than 1/16th of an inch. Aspen Excelsior was the fuel.
In the footnotes, pay particular attention to #2 above. An enlargement is below.
This means that they infused the combustion chamber at the lab with 100cc’s of Nitrogen, i.e. 6.102 cubic inches, per minute to control the reaction. This is stated in this report on page 3 of the original numbering system, or page 8 of the PDF version. I would further point out that the report states as you can see highlighted that they had to develop a new method to test due to the “highly exothermic reaction”. For anyone that simply has never studied this, the term exothermic means:
Exothermic:
In a chemical reaction, this term describes the process of energy (usually in the form of heat) being released from the reaction and transferred to the surroundings.
When the term “highly exothermic” is used, it often means a substantial amount of heat is being released.
And again the test was conducted in an Inert Atmosphere. Okay, so what is an inert atmosphere you may ask? Well, the vacuum of space would be a fine example and is often cited! Or one devoid of oxygen or depleted so much that it cannot support reactions or they (the reactions) can be highly controlled.
Just so we are clear, treating fuels with retardant does NOT create that inert atmosphere, if you read the report you will see they inject 100cc’s or 6.102 cubic inches of Nitrogen per minute to “Control the Reaction”. Sorry but in my mind, this is useless because we simply cannot increase nitrogen to control our reactions of forest fire fuels. Furthermore, if we had any more nitrogen in the atmosphere we’d likely be dead. If the Nitrogen levels were somehow increased to create a pyrolysis effect in wildland fires the oxygen would be displaced to make that happen, hence we’d be dead. How? because it would have to be done on such a global scale this becomes impossible.
The report of INT-90 is here above and online just search for it:
Also I will point out the Pyrolysis elements. This in my view is of little value due to the unwavering fact that we do not live, work, or fight fire in an any sort of Inert Atmosphere nor can we survive in one! Any data concerning such, while beneficial from an academia point, is of little value in the real fire suppression world.
I would finally point out that with regard to all of the hype and talk about how retardants are a critical element of fire suppression work, are highly effective tools, etc., etc., I think we should first understand what the product is before we shove it out the tank. Like everything else in our world, it has limits. We dont even know what they are and forcing folks to use it.
Retardants according to the data found within the reports have some serious issues in my reasoning. From the actual findings:
Testing retardant chemicals for a Pyrolysis effect and being able to show that it has a diminished rate of spread sounds good, however, if you re-read the paper and slow down, you can see that it only works in an inert atmosphere. INT-90 pg 2 & pg 3.
Per INT-90 Thermal analysis in an Oxygen or in an Air atmosphere may or may not accurately represent flaming and glowing combustion. pg 1.
Treating fuels with Ammonium Phosphate chemicals actually lowers the input energy required for ignition, INT-121 pg 1. i.e. the condition of absorbing heat that is called endothermic. The chemical treating allows fuels to ignite at a lower energy input level compared to un-treated fuels.
There is a whole lot more within the documents. You must understand that there are many parts and many factors that are made up within the single report. I again am not advocating we stop using retardants at all, I am however stating that if we want to use the burn inhibiting characteristics of retardants we need to keep the temps below 550C (1,022 F).
How? again, FireBridge. Cool the area immediately before you drop retardant. Get the fuel temps down below the 1,000 deg thresholds.
Burning Colors and associated elements that have been found in Ammonium Phosphate:
Chromium produces Green
Cadmium productes Brown
Arsenic produces Pale Blue
Lead produces Blue-White
Copper produces Blue-Green
Vanadiuim produces Yellow-Green
Manganese produces Yellow-Green
When Ammonia is separated into constituent elements, nitrogen and hydrogen, then introduced to a flame, such as on a wildland fire, the flame color according to various reports is said to be a Yellow to yellowish green.
Antimony produces Pale Green
Barium produces Apple Green-Pale Green
Thalium produces Brilliant Green